ASPIRET Platform
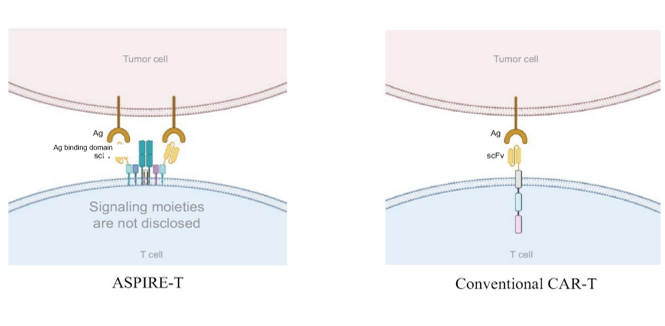
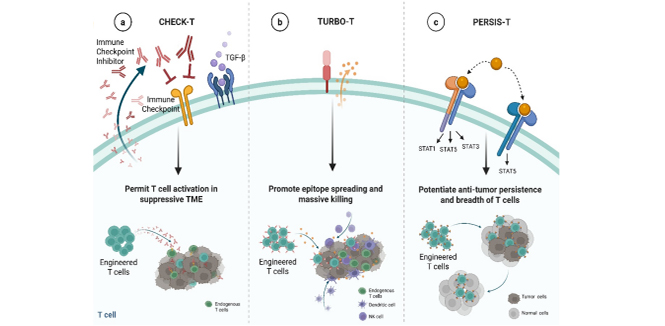
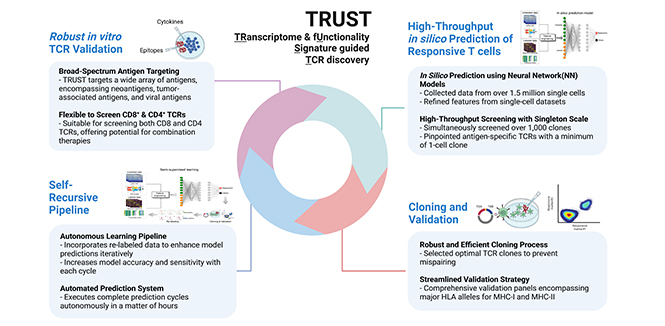
A next-generation T cell therapy platform that redirects the TCR/CD3 complex to overcome key limitations of conventional CAR-T and TCR-T therapies, delivering robust, sustained, and safer immune responses against solid tumors.
A next-generation T cell therapy platform that redirects the TCR/CD3 complex to overcome key limitations of conventional CAR-T and TCR-T therapies, delivering robust, sustained, and safer immune responses against solid tumors.